Matter in Our Surroundings
CONTENT LIST
What is Matter?
Anything that has volume and mass is called matter. Matter in our surrounding are found in three physical states:-
- Solid:- A state of matter that retains its shape and density is called solid.
- Liquid:- A state of matter that doesn't retain it's shape is called liquid. It takes the shape of cointainer.
- Gas:- A state of matter that doesn't retains its shape and density is called gas.
Physical Nature of Matter
- Matter is made up of particles
- The particles of matter are very very small – they are small beyond our imagination!!!!
Characteristics of Matter's praticles
- There is an space between particles of matter, this space is called intermolecular or interparticular space.
- Particles of matter are continuously moving, that is, they have the kinetic energy. When the temperature rises, particles move faster. Thus, we can say that with increase in temperature the kinetic energy of the particles also increases.
- Particles of matter attract each other, particles of matter have force acting between them. This force keeps the particles together. The strength of this force of attraction varies from one kind of matter to another.
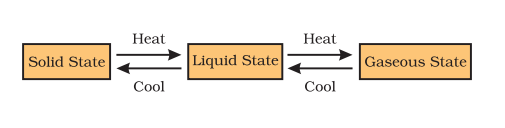
States of Matter
Matter exsist in our surrounding in three forms:-
- Solid
- Liquid
- Gas
Test your understanding
- Perfumes are made up of liquids having boling point. so as soon as it comes in contact with atomspheric temperature it is converted into gas.
- Gas particles have more kinetic energy and less force of attraction between them so they move several meter away.
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
(b) Iron almirah is solid at room temperature is because force of attrction between the particles is more than kinetic energy of particles .
(i) Layers of particles can slip and slide over one another easily.
(ii) Particles just move around randomly because of very weak force of attraction.
(i) Liquid state
(ii) Gaseous state.
Can Matter Change its State?
Matter can change its state by two ways:-
- Change of Temperature
- Effect of pressure
Change of Temperature
The kinetic energy of the particles increases on increasing the temperature of solids. The particles of matter start vibrating with greater speed due to the increase in kinetic energy . The energy supplied through heat source overcomes the forces of attraction between the particles.At this stage of matter, particles leave their fixed positions and start moving more freely and solid melts. Thus matter converted from solid to liquid.
Temperature Scale
There are three scale of temperature measurment:-
- Celcius (°C)
- Farenheite (°F)
- Kelvin (K)
Relation between Celcius and Farenheite
(F-32) * 5/9 = C
F = Temperature in Farenheite scale
C = Temperature in Celcius scale
Relation between Celcius and Kelvin
C + 273 = K
C = Temperature in Celcius scale
K = Temperature in Kelvin scale
Melting point
Melting point is the minimum temperature at which a solid melts to become a liquid at the atmospheric pressure.
The melting point of ice is 273.15 K (Kelvin) or 0°C (degree centigrade) or -32°F(degree forenhite).
The process of melting change matter from solid state into liquid state is also known as fusion.
Temperature of solids remain same during the process of melting means addition of heat to solid during melting does increase the temperture of solid.The supplied heat gets used up in changing the state by overcoming the forces of attraction between the particles.It is called latent heat of meting or fusion
The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
Particles in water at 0°C (273 K) have more energy as compared to particles in ice at the same temperature.
Boiling point
Boiling point is the temperature at which a liquid starts boiling at the atmospheric pressure. Boiling is a bulk phenomenon.
The melting point of ice is 373.15 K (Kelvin) or 100°C (degree centigrade) or 68°F(degree forenhite).
When heat is supply to water, particles start moving even faster. At a certain temperature of water , a point is reached when the water particles have enough energy to overcome the forces of attraction of each other and start moving freely. At this temperature the water starts changing into vapour.This is called the vaporization of water.Temperterture at which water starts vaporizing is boiling point of water.
The latent heat of vaporization of a liquid is the amount of heat required to convert 1 kilogram of the liquid (at its boiling point) to vapour or gas, without any change in temperature.
Water vapour at 373 K (100°C) have more energy than water at the same temperature(100°C) . This is because vapour particles in steam have absorbed extra energy in the form of latent heat of vaporisation.
Matter in our surroundings change state from solid to liquid and from liquid to gas on application of heat.
Effect of pressure
We know that the difference in various states of matter is due to the difference in the intermolecular distances between the constituent particles. Solids have less intermolecular spaces between liquid and liquid have less intermolecular spaces than gas.
When we start putting pressure and compress a gas and reducing tempeture ,It changes their states from gas to ligid and liquid to solid.
Pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
Solid carbon dioxide (CO2)
Solid carbon dioxide (CO2) is stored under high pressure. It gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state and the process is called sublimation. Due to this reason , solid carbon dioxide is also known as dry ice.Test your understanding
Evaporation
The process in which a liquid or solid is converted into vapour at temperature below its boiling point is called evaporation.
Example: Water gets evaporated from the sea surface by the sun’s heating effect.
Differenace between Evaporation and Boiling
- Evaporation takes place only on the surface of the liquid, whereas Boiling process occurs over the large mass of the liquid.
- Bubbling effect is not visible in case of evaporation where it is visible in case of boiling.
- Evaporation is a slow process where as boiling is a fast process.
Factors affecting evaporation
Temperature:
- Temperarture has direct relationships with evaporation rate,on increasing the temperature the rate of evaporation also increases.
- At higher temperatures, the particles near surface of liquid move faster and overcome the force of attraction between the particles and evaporation takes place.
Wind speed:
- Wind speed and rate of evaporation are directly related to each other.
- Higher the wind speed , the rate of evaporation increases.
Surface area:
- The rate of evaporation increases as the surface area increases .
- Water molecules acquire more heat energy from the surroundings if more area is exposed to air.
Humidity:
- Humidity and rate of evaporation are inversly related to each other.
- As the humidity increases, the rate of evaporation decreases.
Test your understanding
1. sea water raising up in day time and making cloud
2. Drying of a mopped floor.
a.Temperature
b. Wind speed
c.Surface area
d. All of these
Related topics of CBSE Science class 9