CONTENT LIST
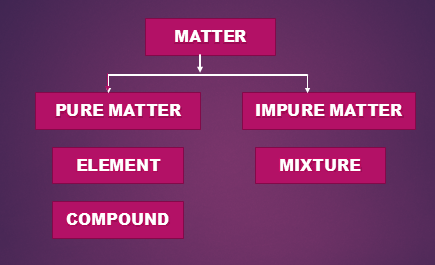
Pure Substance
Pure substance means that all the constituent particles of that substance are same in their chemical nature.
Most of the matter that exist around us are mixtures of two or more pure substances
For example: sea water, minerals, soil etc. are mixtures.
Pure substances can be classified into two categories:
Elements
Elements are pure substance that are made up of similar kind of particles/atoms. Each atoms of element has same chemical properties.
Examples: Hydrogen, Iron, Aluminium etc.
Compounds
Compounds are pure substance that are made up of two or more kinds of particles/atoms combined chemically in fixed ratio.
Examples: water (made up of hydrogen and oxygen atoms in ration of 2:1), Common salt (made up of sodium and chlorine atoms in ratio of 1:1) etc.
Impure Substance
Impure substance means the substance made from two or more than two different kinds of atom. The constituent atom are not chemically combined.
Mixture
Mixture is impure substance that is formed when two or more compounds or elements are mixed in any ratio. Constituent atoms of mixture do not combine chemically.
Types of Mixtures:-
Mixtures are classified into two categories:
- Homogeneous mixtures: In homogeneous mixture the various constituents’ compounds or elements are mixed uniformly.Examples: Salt solution in water, lemon water solution etc.
- Heterogeneous mixtures: In heterogeneous mixtures the various constituents’ compounds or elements are not mixed uniformly. Examples: Concrete is a heterogeneous mixture of stone chips, cement, sand and water.Chocolate chip cookies is an example of heterogeneous mixture.
What is a Solution?
A solution is a homogeneous mixture of two or more substances.
Examples: Lemonade, soda water etc.
Usually we think solution means liquid, it is not correct. There is solid solutions (alloys) and gaseous solutions (air) also.
Any solution has a two components: Solvent and solute
Solvent is the component of the solution that dissolves the other component in it .
(Usually the component present in larger amount) is called the solvent.
Solute is the component of the solution that is dissolved in the solvent (usually present in lesser quantity).
Examples:A solution of sugar water is a solid - liquid solution. In this solution, sugar is the solute and water is the solvent
Properties of a solution
- A solution is a homogeneous mixture.
- The solution particles are smaller than 1 nm in diameter. Therefore they can't be seen by naked eyes.
- Solution do not scatter a beam of light passing through because of very small particle size. Therefore, the path of light is not visible in a solution.
- The solute particles cannot be separated by the process of filtration.
- The solute particles do not settle down when left undisturbed, means a solution is stable.
Concentration of a solution
The amount of solute that is present in a given amount of solution is called concentration of a solution.
It is expressed in terms of:
Mass by the mass percentage of a solution = mass of solute X 100 /mass of solution.
On the basis of amount of solute present in a solution, solution can be named as a dilute, concentrated or a saturated solution.
At any particular temperature, a solution that has dissolved maximum amount of solute as it's capability of dissolving, it is said to be a saturated.
The amount of the solute present in the saturated solution at this temperature is knowns as its solubility.
If the amount of solute present in a solution is less than the saturation level, it is called an unsaturated solution.
(a)Nitrogen Gas
(b)Oxygen Gas
(c)Both (a) and (b)
(d)None of these.
(a) homogeneous
(b) heterogeneous
(c) Both (a) and (b)
(d) pure substance
(a) mixture
(b) compound
(c) element
(d) impure substance
(a) Compound
(b) Element
(c) Homogeneous mixture
(d) Heterogeneous mixture
(a) Sugar is solute, water is solvent
(b) Sugar is solvent, water is solute
(c) Both are solutes
(d) Both are solvents.
(a) solid zinc
(b) molten zinc
(c) gaseous zinc
(d) molten tin
(a) Texture
(b) Solution
(c) Mixture
(d) Compound
(a) Mixture
(b) Texture
(c) Homogenous
(d) Heterogeneous
(a) Alloy
(b) Solution
(c) Mixture
(d) Metallic mixture
(a) Solute
(b) Sugar
(c) Solvent
(d) Mixture
(a) Solute
(b) Sugar
(c) Solvent
(d) Mixture
(a) Heterogeneous
(b) Gas
(c) Solid
(d) Homogenous
(a) 1m
(b) 1cm
(c) 1mm
(d) 1nm
(a) Homogenous
(b) Heterogeneous
(c) Saturated
(d) Unsaturated
What is a suspension?
Suspension is a kind of heterogeneous mixture in which solute particles do not dissolve completely and remain suspended throughout solution and particles of a suspension are visible from naked eye.
The particles of a suspension scatter a beam of light passing through it and make its path visible.
The solute particles settle down when a suspension is left undisturbed for a while that is, a suspension is unstable.
Colloidal
Colloids is also known as colloidal solutions or colloidal systems, these are the mixtures in which microscopically dispersed insoluble particles of one substance are suspended in another substance.
The size of the suspended particles in a colloidal solution ranges from 1 to 1000 nanometres (10^-9 metres).
Colloidal solution is heterogeneous in nature .
Colloidal solutions can not be separated by filtration.
Particles of colloidal are so small that it is not possible to see through naked eyes
Micro particles of colloidal do not settle.
Colloidal particles are bigger than that of the solution but smaller enough to be unseen by naked eyes.
Light passes through colloids shows the Tyndall effect.
Tyndall effect, also known as Tyndall phenomenon, it shows scattering of a beam of light by a medium containing small suspended particles. Example, smoke or dust in a room, which makes light visible as beam entering from a window.
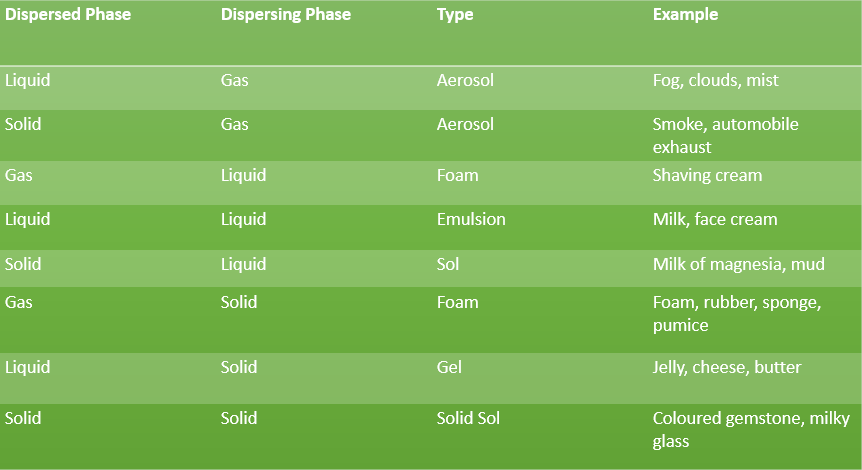
A colloid is made of two components, which include the dispersed phase and the dispersion medium.
Dispersed phase: those substance which are distributed in the dispersing medium in form of colloidal particles are called dispersed phase.
Dispersion phase: Dispersion phase is the medium in which the colloidal particles are dispersed.
Difference between Solution, Suspension, Colliod
Different types of Colloidal
A. Brownian Motion
B. Tyndall Effect
C. Coagulation
D. Electrophoresis
(a)Fog
(b)Jelly
(c)Butter
(d)Song
Separating the Components
Many techanics are adopted to split/separate the substances into independent components of it . Using solvents, Filtration, Sublimation, Magnet separtion, Evaporation, Crystallisation, Distillation, Fractional distillation, Chromatography, Centrifugation, and separating funnel are few physical processes that are commonly used to separate the constituents of mixtures.
In order of learning how to separate mixtures, we will catogorise mixers in three scenarios:
- Mixture of two solids
- Mixture of a solid and a liquid
- Mixture of two liquids
Method of Separation of Components From a Mixture Two Solids
Mixtures containing two solid substances can be separated using following methods:
- Using a suitable solvent
- Using a magnet
- Using the sublimation process
Using a suitable solvent
When one component of the mixture is soluble in a particular liquid solvent and the other component is insoluble in solvent. This difference in solubility of the components of the mixture can be used to separate them by using a suitable solvent.
In order to separate suagar and salt mixture (solid solid mixture), sugar is soluble in water while sand is insoluble in water,therefore water is used as a solvent to separate a mixture of sugar and sand.
Using a magnet
If one component of mixture is magenetic in nature (like iron) then a magnet is used to separate a mixture.
Using the sublimation process
Substances like campher,nepthaline ball etc. change directly from solid-state to vapour state without undergoing into a liquid state, when heated. This process is termed as Sublimation.
To separate a mixture of common salt and ammonium chloride (sublime substance), a sublimation process is used. On heating the mixture, ammonium chloride is converted in to vapour which is further cooled and separate out from mixture.
Method of Separation of Components From a of a Solid and a Liquid
Mixtures containing a solid and a liquid are separated by any one of the following processes:
- By filtration
- By evaporation
- By centrifugation
- By distillation
- By chromatography
- By crystallization
Separation by Filtration
Filtration is the process of removing insoluble solids from a liquid using filter paper. By using this techanique a heterogeneous mixture of solids and liquids can be separated .It separate insoluble substances from liquid.
A mixture of chalk and water is separated by using filtration method.
Separation by evaporation
Evaporation techanique is used to separate solid substances dissolved in water (or other liquids). On heating , all the water (or liquid) evaporates, the dissolved substance remains as a solid residue.
Example: Common salt dissolved in water is separated by the process of evaporation .
This process is also used to obtain coloured components (dye) from ink.
Separation by centrifugation
Centrifugation is a method of separating suspended component of a liquid in which the mixture rotates at high speed in a centrifuge.In centrifugation process, a mixture of fine particles suspended in a liquid is kept in a test tube. The tube is kept in a centrifuge (mechanical device used for centrifugation process) and centrifuge is rotated rapidly for some time. When the mixture rotates on high RPM, the centrifugal force acts on the heavier particles/components of mixture. thus heavier particles/components suspended in mixture are drawn to the test tube’s bottom.
We use this techanique to get many milk products; cream, butter, cheese etc from milk.
Separation by distillation
The process of heating a liquid to form a vapour and then cooling the vapour to recover the liquid is called distillation .The diffrent liquids obtained by condensing the vapour at diffrent lower are called ‘distillate.’
Distillation techanique is used to obtain drinking water from seawater in many countries.
This techaniue is also used to get petrolium products in refinary from crude oil.
Separation by chromatography
This technique is used for separating two (or more) dissolved solids that are present in a solution in very small quantities is called Chromatography . This method is used to separate solutions of coloured substances (dyes and pigments).
Separation by crystallization
Crystallization is a technique to separate components from a liquid mixture.In this process We use of differences in solubility of the components present in the melt or the solution as the basis of separation.
Examples: Crystallization method is used to obtain pure copper sulphate from an impure sample.
Common salt can be purified by this process.
Related topics of CBSE Science class 9