CONTENT LIST
CARBON AND ITS COMPOUNDS
Introduction
The earth’s crust has only 0.02% carbon in the form of minerals (like carbonates,hydrogen carbonates, coal and petroleum) and the atmosphere has 0.03% of carbon dioxide.
Carbon is a common ingredient of substance because it can combine with itself and with many other elements.
There are millions of known carbon compounds, and carbon is the only element that can form so many different compounds.
Bonding in carbon - The covalent bond
Carbon atoms have four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds.
A carbon atom is able to form covalent bonds with other carbon atoms or with the atoms of other elements.
When it bonds only with hydrogen, and forms compounds called hydrocarbons.
Carbon compounds are poor conductors of electricity and have low boiling and melting points due to covelent bond.
Allotropy of carbon
The phenomenon of existence of any element in two or more forms which have different physical properties but identical chemical properties is called allotropy.
The forms of an element have different physical properties but identical chemical properties is known as allotropes.
Carbon has two well known allotropes they are graphite and daimond.
Each carbon atom is bonded to four other carbon atoms In diamond, & forming a rigid three dimensional structure.
Each carbon atom is bonded to three other carbon atoms in the same plane and giving a hexagonal array in graphite,
These two different structures result in diamond and graphite having very different physical properties even though their chemical properties are same.
Diamond is most hardest substance known while graphite is smooth and slippery. Graphite is also a very good conductor of electricity unlike other non-metals.
Versatile nature of carbon
Carbon is one of versatile element and found in many different chemical compounds.
Carbon is so versatile because it can form single, double, and triple bonds. Carbon atom can also form chains, branched chains, and rings when connected to other carbon atoms.
The two important characteristic features of carbon atom , that is, tetravalency and catenation made it unique and due to this carbon atoms form a large number of compounds.
Why Carbon Is Special ?
Carbon is the only element that can form more than ten million organic compounds known by chemists because .
- Carbon is tetravalent element that can form single ,double and triple bond other carbon atom .
- Carbon atom is small size so they can fit comfortably as parts of very large molecules.
- General formula : CnH2n+2
- CH4 = Methane => Four hydrogen atoms are boned through single covelent bond with one cabon atom- Thus carbon atom is fully saturated .
- C2H6 = Ethane = Six hydrogen atoms are boned through single covelent bond with two carbon atoms- Thus carbon atoms are fully saturated
- C3H8 = Propane = Eight hydrogen atoms are boned through single covelent bond with three carbon atoms- Thus carbon atoms are fully saturated
- C4H10 = Butane = Ten hydrogen atoms are boned through single covelent bond with four carbon atoms- Thus carbon atoms are fully saturated
- C5H12 = pentane =Twelve hydrogen atoms are boned through single covelent bond with five carbon atoms- Thus carbon atoms are fully saturated
- C6H14 = hexane = six hydrogen-saturated carbons
- Alkenes
- Alkynes
- Methane, Ethane, and Propane belong to a common homologous series.
- (i) C + O2 → CO2 + heat and light
- (ii) CH4 + O2 → CO2 + H2O + heat and light
- (iii) CH3CH2OH + O2 → CO2 + H2O + heat and light
1.Catenation
Catenation is the ability of carbon atom of binding of an element to itself or with other elements through covalent bonds & form long chains. Carbon atoms are unique because of catenation property.
carbon atom can form straight, branched or cyclic chains by single, double or triple covelent bonds with other carbon atoms.
ii.Small size of Carbon atom
Due to the small size of Carbon atom , they can form compounds with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements.Which is increasing number of compounds with specific properties that depend on the other elements combined with carbon atom in compound.
Saturated and Unsaturated Carbon Compounds
Saturated Carbon Compounds
A saturated organic compounds contain only single bonds between carbon atoms.
Saturated compounds of carbon are called the alkanes.
All alkanes have -ane suffix .
ALKANES :They are also called parrafins.


Unsaturated Carbon Compounds
Hydrocarbons that have double or triple covalent bonds between adjacent carbon atoms is called unsaturated hydrocarbon.
The unsaturated hydrocarbons that contain one or more double bonds are called alkenes.
General formula : CnH2n
The unsaturated hydrocarbons that contains one or more triple bonds are called alkynes.
General formula : CnH2n-2
Isomerism
Carbon atoms can indifferent arrangemnet & form compound , such as long chain, branched chain and cyclic chain structures.
Hydrocarbons which have same molecular formula but different structural formula are called Structural Isomers.
The smallest hydrocarbon that has isomers is butane, which has only four carbon atoms. In the figure shown below structural formulas for normal butane (or n-butane) and its only isomer, called iso-butane.
Both the molecules have four carbon atoms as well as ten hydrogen atoms (C4H10), but atoms are arranged differently in these two compounds. They are called Isomer
In n-butane, four carbon atoms are bonded in a straight chain. In iso-butane, one of the carbon atoms branches off from the main chain.
Normal butane(N-butane)


Iso-butane


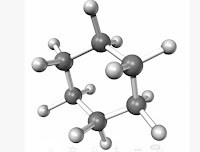
In addition to straight and branched carbon chains, some coarbon compounds have carbon atoms arranged in the form of a ring. For example, cyclohexane has the formula C6H12 and have following structure.
Homologous series
Carbon is a very friendly element.
Carbon atom forms bonds with other elements such as halogens, oxygen, nitrogen and sulphur.
In a hydrocarbon chain, one or more hydrogen atoms can be replaced by the halogens, oxygen, nitrogen and sulphur atom in such way that valency of carbon remains satisfied. In such compounds, the element replacing hydrogen is termed as heteroatom.
A homologous series refers to a group of compounds that share similar properties.
A homologous series is a group of organic compounds that have similar properties & differ from each other by one methylene (CH2 ) group.
Each member of a homologous series is known as a homologue, which is also spelled ''homolog.'' For example, Methane and Ethane are homologues and They belong to the same homologous series. They differ from each other by one CH2 group. The formula of Methane is CH4 and the formula of Ethane is C2 H6.
Functional group
In saturated hydrocarbon , carbon atoms are joined by only a single bond. A saturated hydrocarbon is unreactive, but when another ‘atom’ or ‘group of atoms’ is attached with carbon compound , the resulting molecule becomes highly reactive. The other ‘atom’ or ‘group of atoms’ in a carbon compound is termed as functional group.
An atom or a group of atoms that makes a carbon compound or an organic compound reactive and determines its properties is called functional group .
Some functional groups in carbon compounds
| Hetro atom | Class of compound | Formula |
| Cl/Br | Halo (cholro/Bromo) | -Cl / -Br |
| O (Oxygen) | Alcohol | -OH |
| O (Oxygen) | Aldehyde | -COH |
| O (Oxygen) | Kitone | -C=O |
| O (Oxygen) | Carbocylic acid | -COOH |
Nomenclature of Carbon Compounds
CHEMICAL PROPERTIES OF CARBON COMPOUNDS
Combustion:
Carbon, in all its allotropic forms, burns in oxygen to give carbon dioxide along with the release of heat and light.
Saturated hydrocarbons will generally give a clean flame while unsaturated carbon compounds will give a yellow flame with lots of black smoke.
Oxidation: Oxidation of ethanol in presence of oxidizing agents gives ethanoic acid.

